Statin Tolerance Checker
How This Tool Works
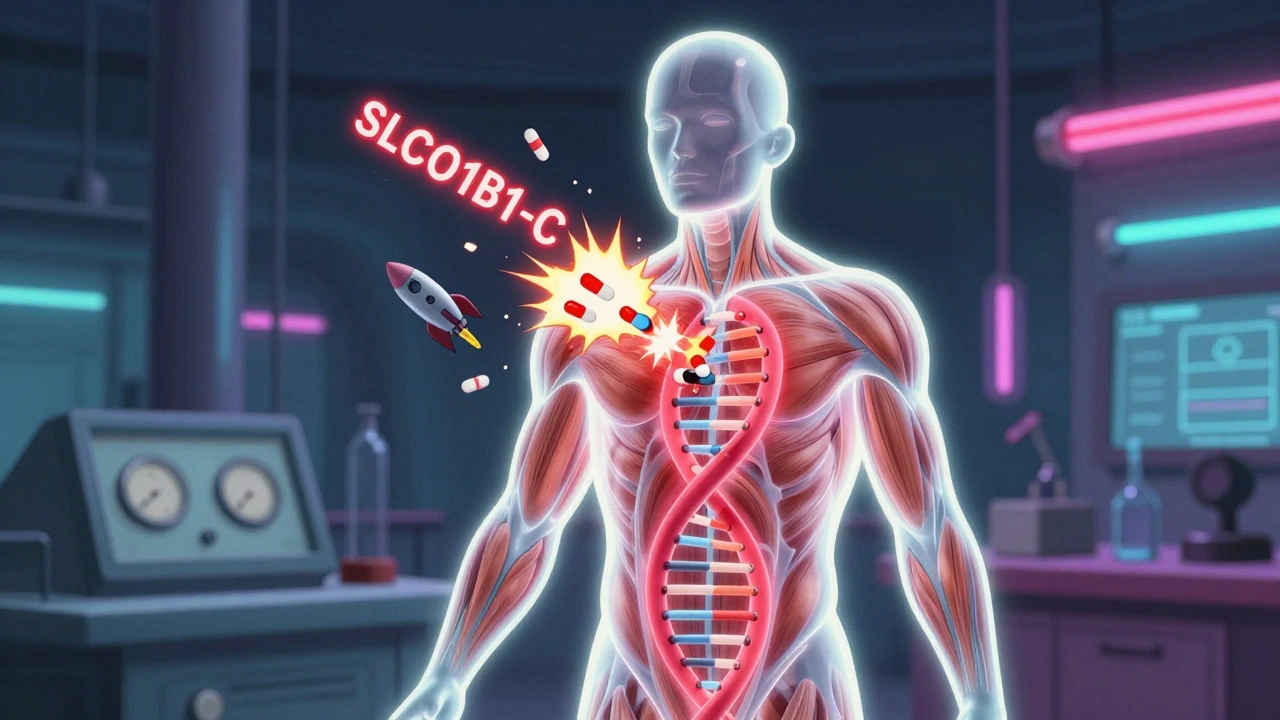
This tool helps determine if a statin medication is safe based on your SLCO1B1 genotype. The SLCO1B1 gene affects how your body processes certain statins, particularly simvastatin. Results are based on CPIC guidelines and clinical evidence.
For millions of people taking statins to lower cholesterol and prevent heart attacks, muscle pain isn’t just an inconvenience-it’s a dealbreaker. About 7% to 29% of patients stop taking statins because of muscle symptoms, even though these drugs save lives. But what if the problem isn’t the drug itself, but your genes? That’s where pharmacogenomics comes in.
Why Some People Can’t Tolerate Statins
Statins work by blocking an enzyme your liver needs to make cholesterol. But they don’t just stay in the liver. Some of the drug gets into your bloodstream and can affect your muscles. For most people, that’s fine. For others, it causes aching, weakness, or cramps-sometimes so bad they quit the medication entirely. The big question: Why do only some people have these side effects? It’s not just age, activity level, or taking other medications. Genetics play a major role. One gene in particular, SLCO1B1, has been linked to how your body handles certain statins. This gene makes a protein that shuttles statins from your blood into your liver. If the gene has a specific variant-called rs4149056 or the C allele-that shuttle doesn’t work as well. The statin builds up in your blood instead, increasing the chance of muscle damage.The SLCO1B1 Gene and Simvastatin
The strongest link between genetics and statin side effects was found with simvastatin, especially at the high 80mg dose. People with two copies of the C variant (CC genotype) are about 4.5 times more likely to develop severe muscle problems than those with two T variants (TT). Even one copy (TC) raises the risk by 2.6 times. These numbers come from large studies published in the New England Journal of Medicine and confirmed by the Clinical Pharmacogenetics Implementation Consortium (CPIC). Here’s what that means in real terms:- CC genotype (1-2% of Europeans): Avoid simvastatin 80mg. Even lower doses can be risky.
- TC genotype (15% of Europeans): Use caution. Lower doses of simvastatin may be okay, but alternatives are safer.
- TT genotype (83% of Europeans): No increased genetic risk. Standard dosing is fine.
Not All Statins Are the Same
Here’s the catch: This genetic risk doesn’t apply to all statins. Rosuvastatin and atorvastatin don’t rely as heavily on the SLCO1B1 transporter. A 2021 study of nearly 12,000 people found no link between the SLCO1B1 variant and muscle symptoms for these two drugs. That’s why CPIC guidelines only cover simvastatin. If you’re genetically at risk, switching statins can make all the difference. Pravastatin and fluvastatin are good alternatives-they’re processed differently and show much lower muscle side effects in people with the CC genotype. One study found pravastatin caused 80% fewer muscle problems in CC carriers compared to simvastatin.
What About Other Genes?
SLCO1B1 is the most proven, but it’s not the whole story. Other genes may play smaller roles:- CYP3A4 and CYP2D6: These help break down statins like atorvastatin and simvastatin. Some people inherit versions that metabolize them slowly, leading to higher blood levels.
- ABCB1 and ABCG2: These control how statins are pumped out of cells. Variants here might trap more drug in muscle tissue.
- GATM and CACNA1S: Linked to muscle metabolism and calcium signaling. Their role is still being studied.
- SOAT1: A newer discovery with strong statistical links to muscle symptoms, but we don’t yet know how it works.
Does Genetic Testing Actually Help?
You might think: If the science is solid, why isn’t everyone getting tested? The answer is complicated. Some studies show real benefits. At Mayo Clinic, 78% of patients with prior statin intolerance were able to restart statin therapy after genetic testing guided their choice. One patient, a 54-year-old woman, switched from simvastatin to pravastatin after testing revealed her CC genotype. Her LDL dropped from 168 to 92 mg/dL-with no muscle pain. But other studies say otherwise. A 2020 trial at Harvard gave SLCO1B1 results to doctors and patients. Did adherence improve? No. Did muscle symptoms drop? Not significantly. Why? Because muscle pain is messy. It’s not always caused by genetics. Stress, vitamin D deficiency, thyroid issues, and even overexertion can mimic statin side effects. And SLCO1B1 only explains about 6% of all statin-related muscle symptoms. That means 94% of cases are due to other factors. Testing can help a subset-but not everyone.Who Should Get Tested?
The American College of Cardiology doesn’t recommend testing for everyone starting statins. But they do say it’s worth considering if:- You had muscle pain with a previous statin and want to try again.
- You’re being considered for high-dose simvastatin (80mg).
- Your family has a history of statin intolerance.

Barriers to Widespread Use
Even with solid science, adoption is slow. Why?- Insurance coverage: Only 28% of private insurers covered SLCO1B1 testing in 2022. Out-of-pocket costs range from $150 to $400.
- Doctor comfort: Only 43% of primary care doctors feel confident interpreting results. Cardiologists do better-82% say they’re comfortable.
- Electronic health record (EHR) integration: Systems like Epic and Cerner can now flag high-risk genotypes when simvastatin is prescribed, but not all clinics use them.
- Confusing reports: Some labs give dense reports. Others give bare-bones results. Without clear guidance, doctors don’t know what to do.
What’s Next?
Researchers are working on better tools. One big push is toward polygenic risk scores-combining SLCO1B1 with other genes to improve prediction. Early data shows this can raise accuracy from 58% to 67%. That’s promising, but not ready for prime time. The Statin Pharmacogenomics Implementation Consortium is now working to standardize testing across 50 U.S. health systems by 2025. Medicare still doesn’t cover it routinely, but that could change as more data comes in.Bottom Line: Is It Worth It?
If you’ve stopped statins because of muscle pain, genetic testing might be your best shot at getting back on them safely. For people with the CC genotype, avoiding high-dose simvastatin can prevent serious muscle damage. Switching to pravastatin or another statin often works. But if you’ve never had side effects, testing probably won’t help. And if your muscle pain persists even after switching statins based on your genes, the issue might be something else entirely. The science is real. The guidelines exist. The tools are available. But this isn’t a magic bullet. It’s a precision tool-for the right person, at the right time.Can pharmacogenomics testing prevent statin side effects?
Yes, but only for a specific group. Testing for the SLCO1B1 gene variant can prevent severe muscle damage in people with the CC genotype who would otherwise take high-dose simvastatin. For them, switching to pravastatin or a lower dose avoids the risk. But testing doesn’t prevent all muscle symptoms, since genetics only explain about 6% of cases. Other factors like age, activity level, and drug interactions matter too.
Which statins are safest for people with SLCO1B1 gene variants?
Pravastatin and fluvastatin are the safest options for people with the SLCO1B1 CC or TC genotype. These statins don’t rely heavily on the OATP1B1 transporter, so they build up less in the blood. Rosuvastatin and atorvastatin are also generally safe, since studies show no strong link between SLCO1B1 variants and their side effects. Simvastatin, especially at 80mg, is the highest risk.
How much does pharmacogenomic testing for statins cost?
Standalone SLCO1B1 testing typically costs between $150 and $400 out-of-pocket. Insurance coverage is limited-only about 28% of private insurers covered it in 2022. Some health systems offer it for free as part of pre-emptive pharmacogenomic programs. Medicare rarely covers it unless it’s part of a specific approved program. Always check with your provider before testing.
Should I get tested before starting a statin?
Not for everyone. Routine testing before first-time statin use isn’t recommended by major guidelines. But if you have a family history of statin intolerance, or you’re at high risk for heart disease and need a strong statin like simvastatin, pre-emptive testing may help avoid future problems. Studies show pre-emptive testing improves adherence by nearly 20% compared to testing after side effects occur.
Do I need to retake the test if I switch statins?
No. Your SLCO1B1 genotype doesn’t change. Once you’ve been tested, the result is lifelong. If you switch statins later, your doctor can refer back to your genetic profile. That’s why some clinics do pre-emptive testing for multiple drugs at once-not just statins, but also blood thinners and antidepressants.

Julia Jakob
December 3, 2025 AT 18:56so like... my aunt took simvastatin and swore her legs felt like wet noodles? turns out she’s CC genotype. switched to pravastatin and now she hikes 10 miles on weekends. genetics aren’t magic but they’re way more useful than my doctor’s guesswork 🤷♀️
Robert Altmannshofer
December 4, 2025 AT 19:26man i wish this was common knowledge when i got kicked off my statin 5 years ago. thought i was just lazy. turns out my body was basically screaming ‘stop, this isn’t you’.
now i’m on rosuvastatin, no issues, and my cholesterol’s under control. why aren’t we doing this for everyone? it’s not like it’s expensive compared to a heart attack.
Kathleen Koopman
December 5, 2025 AT 03:42😭 this is the kind of info i wish my doctor had handed me on a sticky note. i’ve been off statins for 3 years because of cramps. just found out i’m TC. going to ask for pravastatin next visit. thank you for writing this.
Nancy M
December 6, 2025 AT 12:31in my country, we don’t even have access to this testing. my dad had a bad reaction, and they just told him to ‘try a different one’-no gene talk, no guidance. it’s frustrating when science is ready but systems aren’t.
we need policy change, not just patient luck.
Melania Dellavega
December 8, 2025 AT 08:27it’s funny how we treat genetics like a secret code only specialists can crack. but the truth is, your DNA doesn’t care about your insurance plan or your doctor’s comfort level.
if a simple swab can keep someone from quitting a life-saving drug, why are we making it this hard? this isn’t niche science-it’s basic patient care.
we’re not just treating cholesterol. we’re treating people who’ve been told their pain is ‘in their head’ for years.
Shannon Wright
December 10, 2025 AT 00:35As someone who works in clinical education, I’ve seen firsthand how this information transforms patient outcomes-when it’s delivered properly. The issue isn’t the science; it’s the delivery system.
Doctors need training, EHRs need smart alerts, and patients need plain-language summaries-not jargon-filled lab reports. At our hospital, we now embed pharmacogenomic results directly into the EHR with color-coded risk indicators. Adherence jumped 22% in the first year.
It’s not about testing everyone. It’s about testing the right people at the right time-with support. And yes, insurance needs to catch up. But the bigger barrier? Complacency. We’ve accepted muscle pain as ‘just part of the deal’ when it’s actually a genetic red flag.
Imagine if we treated every side effect like a data point instead of a reason to quit. We’d save lives, reduce hospitalizations, and stop blaming patients for ‘non-compliance’ when the real issue was a mismatch between their biology and their prescription.
This isn’t futuristic medicine. It’s precision medicine. And it’s already here. We just need the will to use it.
gladys morante
December 11, 2025 AT 07:34you know what’s worse than statin side effects? being told your pain isn’t real because your genes ‘don’t explain it all’. i’ve been dismissed so many times i stopped going to the doctor.
now i’m on a plant-based diet and fish oil. my cholesterol’s ‘fine’-according to the numbers. but my body? still screaming.
Precious Angel
December 12, 2025 AT 12:33let’s be real-this whole pharmacogenomics thing is just Big Pharma’s new way to sell tests. they know most people won’t understand the data, so they’ll pay $300 for a ‘personalized’ answer that doesn’t change anything.
and don’t get me started on ‘polygenic risk scores’-that’s just statistical voodoo wrapped in a lab coat. they’ll test you for 50 genes, say you’re ‘at risk’ for everything, and then sell you supplements.
the truth? statins are toxic. they mess with your mitochondria. your muscles hurt because your cells are starving. no gene variant changes that. it’s just a distraction from the real problem: overmedication.
if you want to lower cholesterol, eat less sugar. walk more. sleep better. not take a pill your body wasn’t meant to process.
Rachel Nimmons
December 12, 2025 AT 15:00they’re tracking your genes now. next they’ll be tracking your sleep, your stress, your grocery receipts. soon they’ll say your statin intolerance was caused by your instagram scrolling. don’t be surprised if your insurance denies coverage because your ‘lifestyle risk score’ was too low.